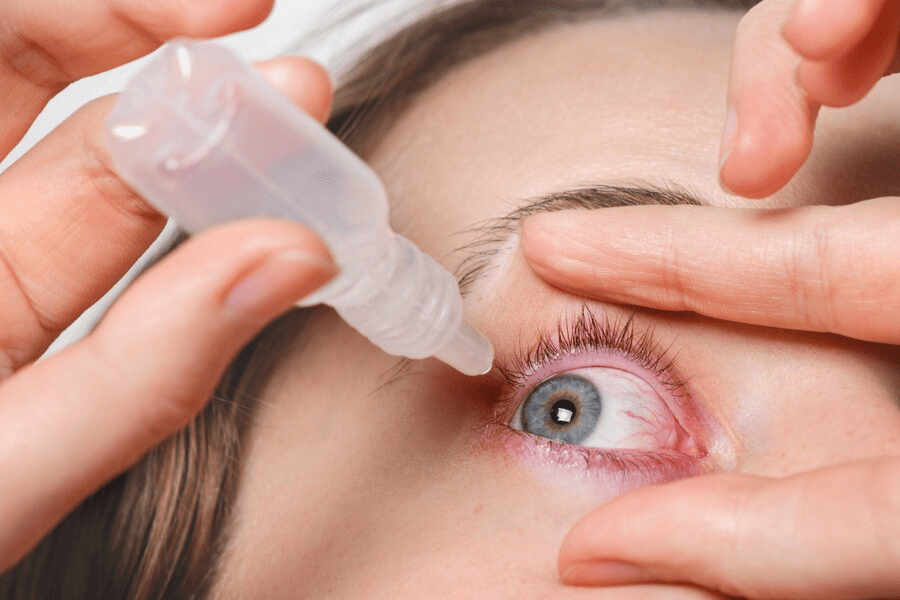
In a recent and alarming development, the Food and Drug Administration (FDA) has issued a warning concerning infections from eye drops that have been detected in the United States. Eye infections can cause significant discomfort and pose serious risks to one’s vision, making this warning a matter of utmost importance for all individuals who rely on eye drops to maintain their ocular health.
The Alarming Rise of Infections from Eye Drops
Recent reports have indicated a concerning increase in eye infections linked to the use of over-the-counter eye drops, including both critical drug production areas and well-known brands. Eye infections can manifest in various forms, ranging from redness and discomfort to more severe complications, such as partial vision loss.
FDA’s Vigilant Monitoring
The FDA, as the nation’s authority in ensuring the safety and efficacy of pharmaceuticals, has been vigilant in monitoring the situation. The regulatory body has reported multiple cases of infections related to eye drops, often caused by drug-resistant bacteria and contaminated eye drops.
Risk of Infected Eye Drops
Infections due to eye drops can occur due to bacterial contamination, which can be detrimental to your eye health. The FDA warns consumers to be aware of the potential heightened risk associated with using these products. If you are experiencing any symptoms of eye infections, such as blurry vision, seek medical care immediately, as prompt attention is crucial in preventing further complications.
Brands at Risk
Several major brands have been flagged for potential bacterial contamination in their ophthalmic drug products. This contamination can lead to a host of adverse events, including partial vision loss. Among the brands mentioned are Rite Aid, Target, and CVS Health, all of which have been identified as potentially affected by this issue.
On October 30, 2023, the FDA revised the list of over-the-counter eye drop products that consumers should avoid buying or using. This update now encompasses Equate Hydration PF Lubricant Eye Drop 10 mL, which is available both in stores and online.
The Implications of Bacterial Contamination
Bacterial contamination is a significant concern, as it can introduce harmful microorganisms into the eye, leading to a range of health issues. Among the most alarming discoveries are positive bacterial test results for eye drops distributed by Cardinal Health and Velocity Pharma, potentially exposing users to harmful bacteria like Pseudomonas aeruginosa.
FDA’s Stern Warning to Consumers
The FDA issued a warning on October 27, 2023, advising consumers against buying and urging them to discontinue the use of certain eye drop products. This caution stems from the possible threat of an eye infection, which could lead to partial vision loss or even blindness. Individuals experiencing signs or symptoms of an eye infection following the use of these items should consult their healthcare provider promptly. These drugs are available under the following brands CVS Health, Leader (Cardinal Health), Rugby (Cardinal Health), Rite Aid, Target Up & Up, Velocity Pharma.
The FDA is not taking this matter lightly. They have issued a strong warning to consumers, emphasizing the importance of seeking medical care if they experience any adverse events related to the use of these products. Bacterial infections can weaken the body’s natural defenses, leaving individuals vulnerable to more severe eye conditions and complications.
Critical Actions to Take
For those who have used eye drops from the affected brands, it is vital to monitor your eye health closely. If you experience symptoms such as redness, pain, or partial vision loss, seek immediate medical attention. The earlier you address these concerns, the better your chances of preventing further damage to your vision.
FDA’s Response to the Crisis
The FDA is actively working to address the issue of bacterial and fungal contamination in ophthalmic drug products. They have urged manufacturers to enhance their quality control processes and take necessary measures to prevent further contamination. This includes conducting more stringent testing to ensure the safety of their products.
Legal Implications and Seeking Guidance
For individuals who have experienced adverse events or eye infections linked to contaminated eye drops, seeking legal guidance is a prudent step. Those affected may have grounds to pursue legal action against the manufacturers and distributors of the affected products.
In such cases, you need to consult with a legal professional who specializes in product liability and personal injury claims. These experienced attorneys can help victims of contaminated eye drops understand their rights and explore potential legal actions to seek compensation for their injuries and losses.

Consult with a Personal Injury Attorney at BLG
The FDA’s warning regarding infections due to eye drops is a stark reminder of the importance of ensuring the safety of our healthcare products. With the potential for bacterial and fungal contamination in ophthalmic drug products, consumers must exercise caution and seek prompt medical care if they experience any adverse events related to these products.
Those who have been affected by infections due to eye drops, need to seek legal guidance to explore potential avenues for compensation and justice. As the FDA continues to investigate and address this issue, you must stay informed and take proactive steps to protect your eye health. Your vision is invaluable, and your well-being should never be compromised by contaminated eye drops.
If you’ve been affected by eye drop-related infections, consult a personal injury attorney at BLG today. Our legal experts specialize in product liability and personal injury claims, and we’re here to help you understand your rights and explore potential legal actions for compensation.
The most current list can be found here: https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-consumers-not-purchase-or-use-certain-eye-drops-several-major-brands-due-risk-eye
Contact us now for a free consultation!





